Structure Of An Atom Resembles Our Solar System!
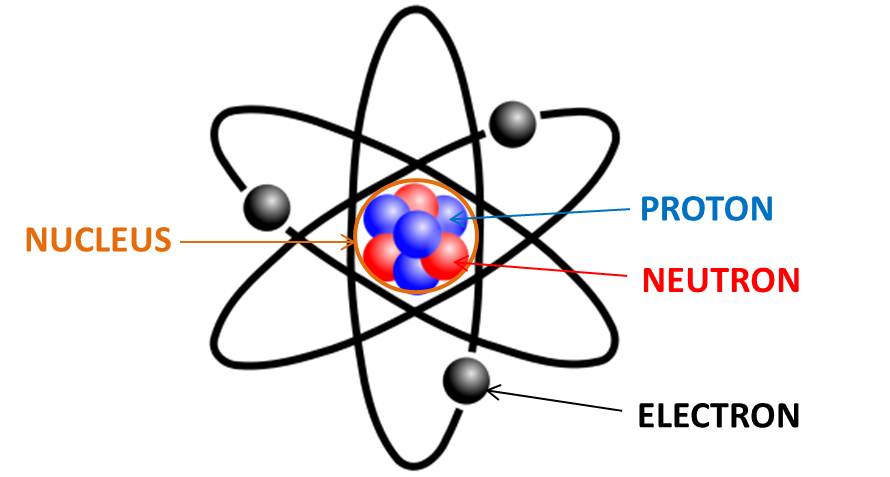

Anything that occupies space and has weight is called matter. The basic unit of matter is an atom. The word atom is coined from a Greek word which means indivisible. People once thought that the tiniest particle in the universe which could not be divided were atoms. The atom consists of subatomic particles. When JJ. Thomson proposed the model called the plum pudding model in the year 1897, the model explained the presence of electrons during cathode ray experiment. Rutherford conducted experiments to study the structure of an atom with the help of alpha particles. He concluded that electrons orbit around a center point. This center is the nucleus of the atom and is positively charged particle. Therefore he concluded that the atom consists of subatomic particles which have negatively charged particles(electrons) orbiting around the nucleus consisting of positively charged particles and the structure of an atom resembles that of our solar system. Hence it is known as Rutherford Atomic Model.
Ernest Rutherford, who was a physicist from New Zealand conducted a gold foil experiment with his companions Hans Geiger and Ernest Marsden. He said if the plum pudding model is true then when alpha particles are focused on the foil it should pass through it in a straight line only. Below are the details of the experiment which was performed in the dark. The experiment was conducted in the dark so that the alpha particles could be clearly observed.
A gold foil of 1000 atoms thick was taken. A radioactive source was arranged to focus high-velocity alpha particles on the gold foil. Majority of the particles passed through the foil undeflected, few showed slight deflection from the expected path and few in the opposite direction. Therefore he concluded that:
- As there is a deflection of alpha particles, there is a positive charge in the atom and called it the nucleus
- Majority of alpha particles passed through the foil undeflected in a straight line. Therefore the atom is mostly empty space
- As some alpha particles traveled back from the foil, the atom’s mass is carried by the nucleus
This experiment demonstrated to study the structure of an atom, explained that the atom consists of subatomic particles. These particles are positively and negatively charged. The positively charged particles are at the center and the negatively charged particles orbit around it. The positive charge at the center is called the nucleus of the atom and the subatomic particles around it are called electrons. Like the planets orbiting around the sun (center of our solar system), electrons move around the nucleus(center of an atom) and hence is also known as the Planetary Model of Atom.
If you find this interesting and would love to know various other concepts like the Tyndall effect, Chemical thermodynamics, plum pudding model by JJ Thomson, mole concept, etc. visit BYJU’S – India’s largest k-12 learning app. For a better understanding of chemistry concepts, you can subscribe to BYJU’S YouTube channel and learn in a more engaging and effective way




